COVID-19 N95 Masks…The Tech Behind It and its Origins !!!

N95 Masks
The COVID-19 is the most talked thing talked about in recent times and that’s how important it has become, and with that one thing that gained popularity is the N95 Mask.Let’s dig deeper into the tech behind it and it’s origins.
With the advent of a novel H1N1 influenza outbreak in spring 2009 and the expectation of a second wave during the 2009–2010 flu season, there has been considerable interest in the use of surgical masks (face masks) and respirators as infection control measures. Although their appearance is often similar, respirators are designed and engineered for distinctly different functions than surgical masks. The amount of exposure reduction offered by respirators and surgical masks differs. The National Institute for Occupational Safety and Health (NIOSH) and the Centers for Disease Control and Prevention (CDC) recommend the use of a NIOSH-certified N95 or better respirator for the protection of healthcare workers who come in direct contact with patients with H1N1.
The N95 mask is a descendant of Wu’s design. Through World War I and World War II, scientists invented air-filtering gas masks that wrapped around your entire head to clean the air supply. Similar masks, loaded with fiberglass filters, began to be used in the mining industry to prevent black lung.
This equipment saved lives, but it was burdensome, and a large reason why were the filters. The fiberglass required a lot of effort to breathe, and the full head enclosures were hot to wear. By the 1950s, scientists began to understand the dangers of inhaling asbestos, but people working with asbestos preferred not to wear bulky respirator masks. Imagine working in construction in 85-degree heat and having your head wrapped in rubber to protect yourself from an invisible threat.
So in the 1970s, the Bureau of Mines and the National Institute for Occupational Safety and Health teamed up on creating the first criteria for what they called “single use respirators.” The first single-use N95 “dust” respirator as we know it was developed by 3M, according to the company, and approved on May 25, 1972. Instead of fiberglass, the company repurposed a technology it had developed for making stiffer gift ribbons into a filter, by taking a melted polymer and air-blasted it into layers of tiny fibers.As particles, whether silica or viruses, fly into this maze of sticks, they get stuck making turns. 3M also added an electrostatic charge to the material, so even smaller particles find themselves pulled toward the fibers. Meanwhile, because there are so many big holes, breathing is easy.
The longer you wear an N95 respirator, the more efficient it becomes at filtering out particles. More particles just help filter more particles. But breathing becomes more difficult over time as those gaping holes between the fibers get clogged up with particles, which is why an N95 respirator can’t be worn for more than about eight hours at a time in a very dusty environment. It doesn’t stop filtering; it just prevents you from breathing comfortably.
N95 respirators were used in industrial applications for decades before the need for a respirator circled back to clinical settings in the 1990s with the rise of drug-resistant tuberculosis. HIV had a lot to do with its spread across immunocompromised patients, but tuberculosis infected many healthcare workers, too. To stop its airborne spread, N95 standards were updated for healthcare settings, and doctors began wearing them when helping tuberculosis patients. Even still, respirators are rarely used in hospitals to this day because it’s only outbreaks like COVID-19 that necessitate so much protection.
Wu went on to found China’s version of the CDC, narrowly miss winning a Nobel Prize, and be featured in many biographies (including his own autobiography). More recently, during the SARS outbreak, people in China wore facial protection to prevent the spread of illness. Then as pollution took over cities like Beijing, they wore respirators to filter pollution.
The N95 respirator isn’t perfect. It isn’t designed to seal well to the face of children or those with facial hair, and if it doesn’t seal, it doesn’t work as advertised. Furthermore, the N95 variants that are worn in high-risk operating rooms don’t have an exhalation valve, so they can get particularly hot to wear.
But the N95 respirator evolved over hundreds of years in response to multiple crises. That evolution will only continue through and beyond the COVID-19 pandemic.

Filtration Process
Filter Performance
The filters used in modern surgical masks and respirators are considered “fibrous” in nature—constructed from flat, nonwoven mats of fine fibers. Fiber diameter, porosity (the ratio of open space to fibers) and filter thickness all play a role in how well a filter collects particles. In all fibrous filters, three “mechanical” collection mechanisms operate to capture particles: inertial impaction, interception, and diffusion. Inertial impaction and interception are the mechanisms responsible for collecting larger particles, while diffusion is the mechanism responsible for collecting smaller particles. In some fibrous filters constructed from charged fibers, an additional mechanism of electrostatic attraction also operates. This mechanism aids in the collection of both larger and smaller particle sizes. This latter mechanism is very important to filtering facepiece respirator filters that meet the stringent NIOSH filter efficiency and breathing resistance requirements because it enhances particle collection without increasing breathing resistance.
How do filters collect particles?

Filtration Mechanisms
These capture, or filtration, mechanisms are described as follows:
Figure 1: Filtration mechanisms
- Inertial impaction: With this mechanism, particles having too much inertia due to size or mass cannot follow the airstream as it is diverted around a filter fiber. This mechanism is responsible for collecting larger particles.
- Interception: As particles pass close to a filter fiber, they may be intercepted by the fiber. Again, this mechanism is responsible for collecting larger particles.
- Diffusion: Small particles are constantly bombarded by air molecules, which causes them to deviate from the airstream and come into contact with a filter fiber. This mechanism is responsible for collecting smaller particles.
- Electrostatic attraction: Oppositely charged particles are attracted to a charged fiber. This collection mechanism does not favor a certain particle size.
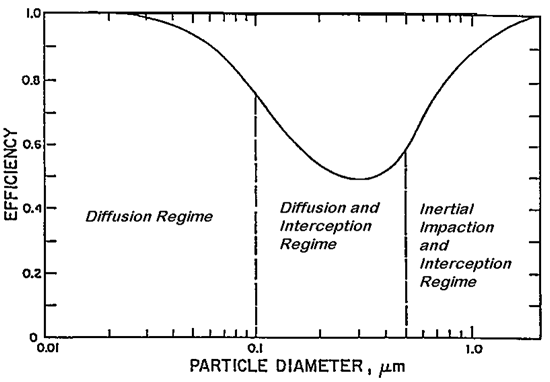
In all cases, once a particle comes in contact with a filter fiber, it is removed from the airstream and strongly held by molecular attractive forces. It is very difficult for such particles to be removed once they are collected. As seen in Figure 2, there is a particle size at which none of the “mechanical” collection mechanisms (interception, impaction, or diffusion) is particularly effective. This “most penetrating particle size” (MPPS) marks the best point at which to measure filter performance. If the filter demonstrates a high level of performance at the MPPS, then particles both smaller AND larger will be collected with even higher performance.
This is perhaps the most misunderstood aspect of filter performance and bears repeating. Filters do NOT act as sieves. One of the best tests of a filter’s performance involves measuring particle collection at its most penetrating particle size, which ensures better performance for larger and smaller particles. Further, the filter’s collection efficiency is a function of the size of the particles, and is not dependent on whether they are bio aerosols or inert particles.
How are surgical masks and respirator filters tested?
Respirator filters must meet stringent certification tests (42 CFR Part 84) established by NIOSH. The NIOSH tests use what are considered “worst case” parameters, including:
- A sodium chloride (for N-series filters) or a dioctyl phthalate oil (for R- and P-series filters) test aerosol with a mass median aerodynamic diameter particle of about 0.3 µm, which is in the MPPS-range for most filters
- Airflow rate of 85 L/min, which represents a moderately-high work rate
- Conditioning at 85% relative humidity and 38°C for 24 hours prior to testing
- An initial breathing resistance (resistance to airflow) not exceeding 35 mm water column* height pressure and initial exhalation resistance not exceeding 25 mm water column height pressure
- A charge-neutralized aerosol
- Aerosol loading conducted to a minimum of 200 mg, which represents a very high workplace exposure
- The filter efficiency cannot fall below the certification class level at any time during the NIOSH certification tests
* Millimeters (mm) of water column is a unit for pressure measurement of small pressure differences. It is defined as the pressure exerted by a column of water of 1 millimeter in height at defined conditions, for example 39°F (4°C) at standard gravity.
As a result of these stringent performance parameters, fiber diameters, porosity, and filter thicknesses of all particulate filters used in NIOSH-certified respirators, including N95s, are designed and engineered to provide very high levels of particle collection efficiencies at their MPPS.
Manufacturers of surgical masks, on the other hand, must demonstrate that their product is at least as good as a mask already on the market to obtain “clearance” for marketing. Manufacturers may choose from filter tests using a biological organism aerosol at an airflow of 28 L/min (bacterial filtration efficiency) or an aerosol of 0.1 µm latex spheres and a velocity ranging from 0.5 to 25 cm/sec (particulate filtration efficiency). It is important to note that the Food and Drug Administration specifies that the latex sphere aerosol must not be charge-neutralized.
The generation of the test aerosol can impart a charge on a higher percentage of the aerosolized particles than may normally be expected in workplace exposures. A charge-neutralized test aerosol, like those used in the NIOSH tests, has the charges on the aerosolized particles reduced to an equilibrium condition. Therefore, higher filter efficiency values than would be expected with the use of charge-neutralized aerosols may result due to the collection of charged particles by the filters’ electrostatic attraction properties. Additionally, allowing the manufacturer to select from a range of air velocity means that the test results can be easily manipulated. In general, particles are collected with higher efficiency at lower velocity through a filter.
Both of these aspects yield a test that is not necessarily “worst case” for a surgical mask filter. Because the performance parameters for surgical masks are less stringent than those required for filters used in NIOSH-certified respirators, the fiber diameters, porosity, and filter thicknesses found in surgical masks are designed with significantly lower levels of particle collection efficiencies at their MPPS.
How do surgical mask and respirator filters perform?
Respirator filters that collect at least 95% of the challenge aerosol are given a 95 rating. Those that collect at least 99% receive a “99” rating. And those that collect at least 99.97% (essentially 100%) receive a “100” rating. Respirator filters are rated as N, R, or P for their level of protection against oil aerosols. This rating is important in industry because some industrial oils can remove electrostatic charges from the filter media, thereby degrading (reducing) the filter efficiency performance. Respirators are rated “N” if they are not resistant to oil, “R” if somewhat resistant to oil, and “P” if strongly resistant (oil proof). Thus, there are nine types of particulate respirator filters:
- N95, N-99, and N-100
- R-95, R-99, and R-100
- P-95, P-99, and P-100
Respirator filters are tested by NIOSH at the time of application and periodically afterward to ensure that they continue to meet the certification test criteria. The FDA does not perform an independent evaluation of surgical mask filter performance, nor does it publish manufacturers’ test results. In many cases it is difficult to find information about the filter test results for FDA-cleared surgical masks. The class of FDA-cleared surgical masks known as Surgical N95 Respirators is the one clear exception to this uncertainty of filter performance. This is the only type of surgical mask that includes evaluation to the stringent NIOSH standards. All members of this class of surgical masks have been approved by NIOSH as N95 respirators prior to their clearance by the FDA as surgical masks. The FDA, in part, accepts the NIOSH filter efficiency and breathing resistance test results as exceeding the usual surgical mask requirements.
In studies comparing the performance of surgical mask filters using a standardized airflow, filter performance has been shown to be highly variable. Collection efficiency of surgical mask filters can range from less than 10% to nearly 90% for different manufacturers’ masks when measured using the test parameters for NIOSH certification. Published results on the FDA-required tests (if available) are not predictive of their performance in these studies.
It is important to keep in mind that overall performance of any facepiece for particulate filtering depends, first, on good filter performance. A facepiece or mask that fits well to the face but has a poor filter will not be able to provide a high level of protection.
The technology behind these masks are evolving over time , but we all hope that we will not have the need to wear them in near future


Have you ever thought about adding a little bit more than just your articles?
I mean, what you say is valuable and everything.
Nevertheless think of if you added some great visuals or videos
to give your posts more, “pop”! Your content is excellent but
with images and video clips, this website could undeniably be one of the most beneficial in its niche.
Fantastic blog!
thanks, for the feedback, we will definitely look into it